Nanobody Cheat Sheet
We developed a nanobody cheat sheet that is handy for antibody-related work. Below we outline specific sections of the guide, but you can download the pdf in its entirety after subscribing to our newsletter. You can unsubscribe at any time!
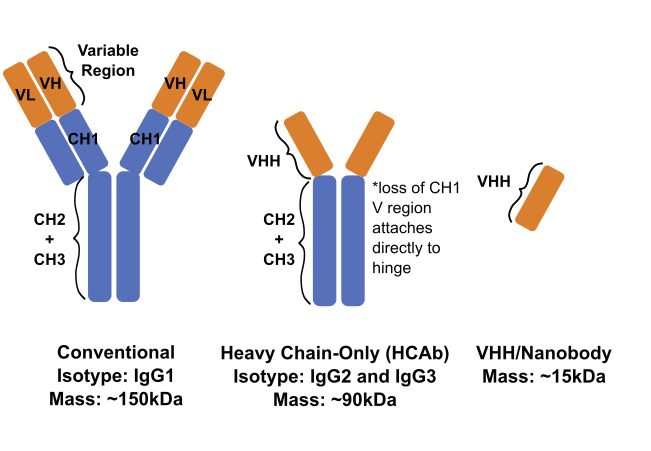
Antibody Structures
Camelids produce two types of antibodies: conventional antibodies and heavy chain-only antibodies.
- Conventional Antibodies – Consists of two identical heavy chains and two identical light chains. Can comprise 20%-75% of the total Ig response to an immunogen.
- Heavy Chain-only Antibodies – Consists of two identical heavy chains. Due to a deletion of the CH1, there is no bond with a light chain.
- VHHs or Nanobodies – The variable region of heavy chain-only antibodies can be isolated via enzymatic digestion or recombinant expression as a single polypeptide.
Further reading:
Hamers-Casterman, C. T. S. G., et al. “Naturally occurring antibodies devoid of light chains.” Nature 363.6428 (1993): 446-448.
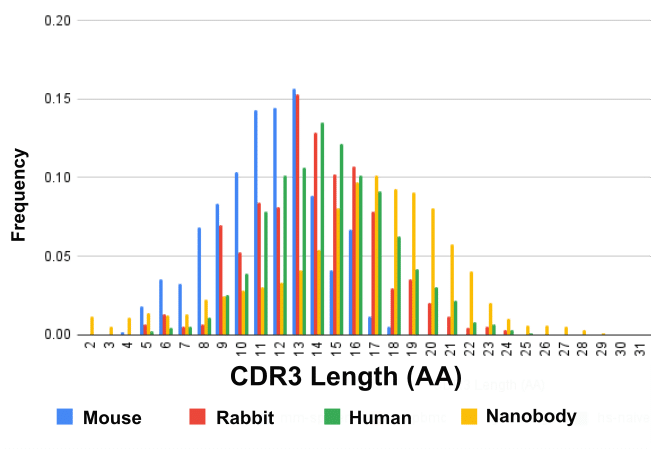
CDR3 Length
The heavy chain-only antibody repertoire exhibits longer CDR3 loops than the heavy chains of other species. Below are the median CDR3 lengths in amino acids across species commonly used in antibody discovery.
- Mouse H-CDR3: 11 amino acids
- Rabbit H-CDR3: 13 amino acids
- Human H-CDR3: 14 amino acids
- Llama HCAb CDR3: 16-17 amino acids
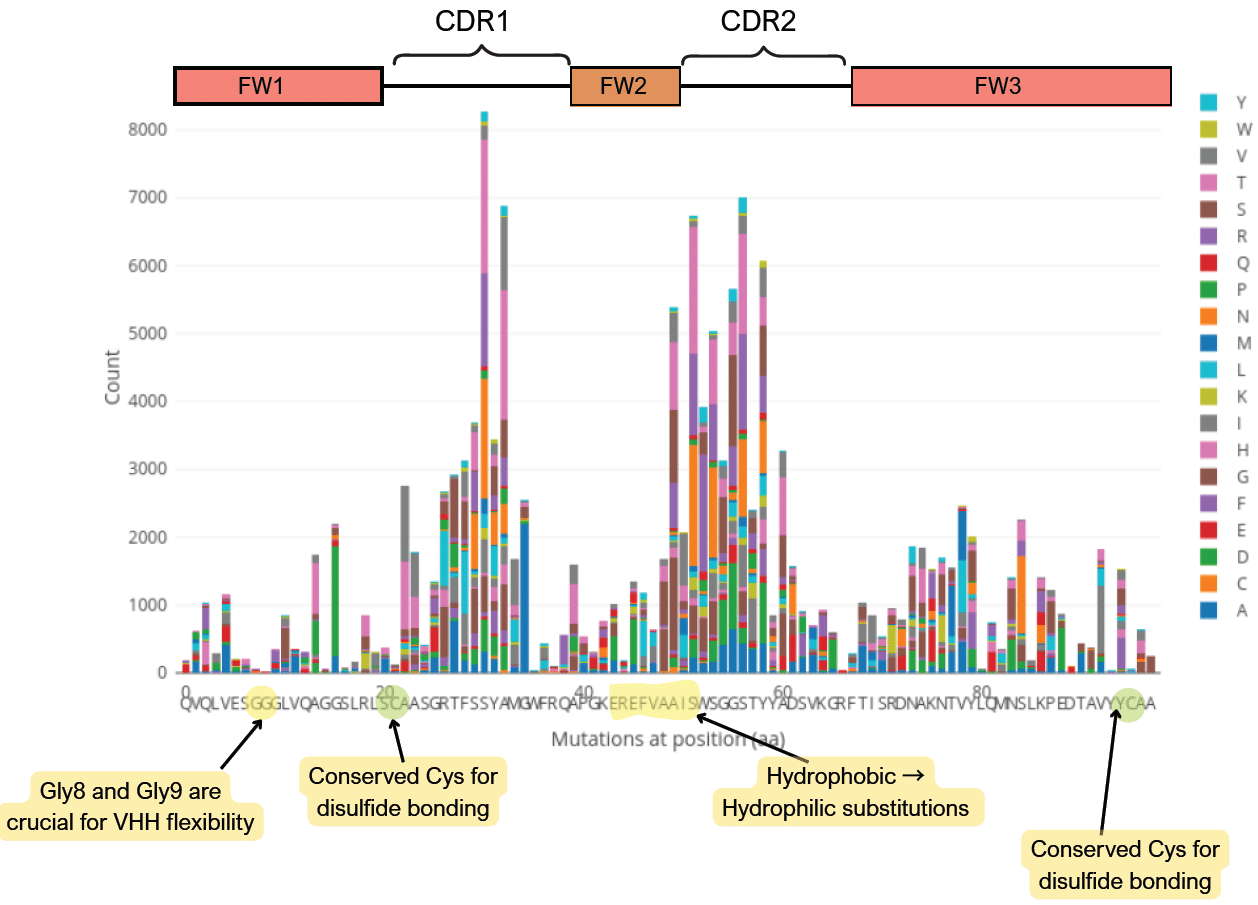
Common Mutations in Nanobodies
The absence of a a light associated with a Nanobody results in structural and sequence differences as compared to conventional antibodies.
- In FW2-CDR3, there is a hydrophobic patch in conventional antibodies where the light chain typically blocks. Without a light chain, these residues are more likely to be hydrophilic in Nanobodies.
- Nanobodies have a higher mutation rate than conventional antibodies in other species.
