Antibody Reference Guide
We developed a one-sheet antibody reference that is handy for antibody-related work. We refer to it often to remind ourselves of the major antibody isotypes, antibody fragments, and purification strategies, as well as antibody development processes. Below we outline specific sections of the antibody reference guide, but you can download the pdf in its entirety after subscribing to our newsletter. You can unsubscribe at any time!
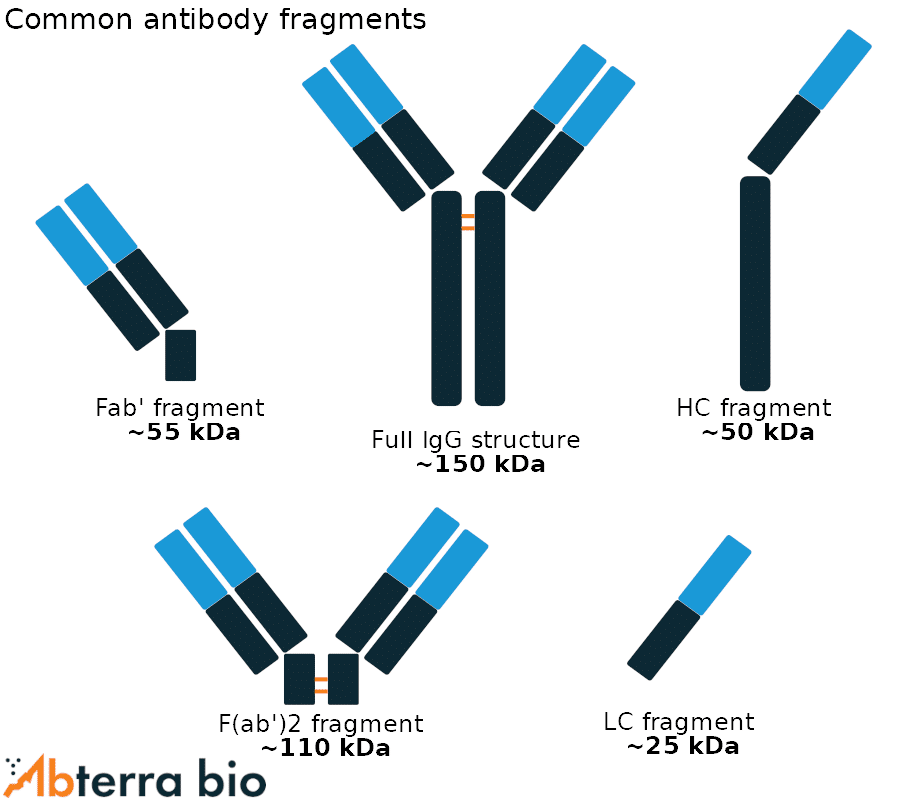
Common antibody fragments
Human antibodies are tetramers, comprised of two identical heavy chains and two identical light chains linked by disulfide bridges. The antibodies can be decomposed into a variety of fragments shown at right, these include:
- Fab’ fragment – contains the variable regions of both the heavy and the light chain and can be recovered using enzymes, such as pepsin. Crucially, the enzymes cleave above the disulfide bridges linking the heavy chains.
- F(ab’)2 fragment – nearly identical to the Fab’ fragment except the cleavage occurs below the disulfide bridge joining the heavy chains, and two identical antigen-binding domains are present.
- HC/LC fragment – the heavy chain and light monomers can be purified under reducing conditions.
Further reading:
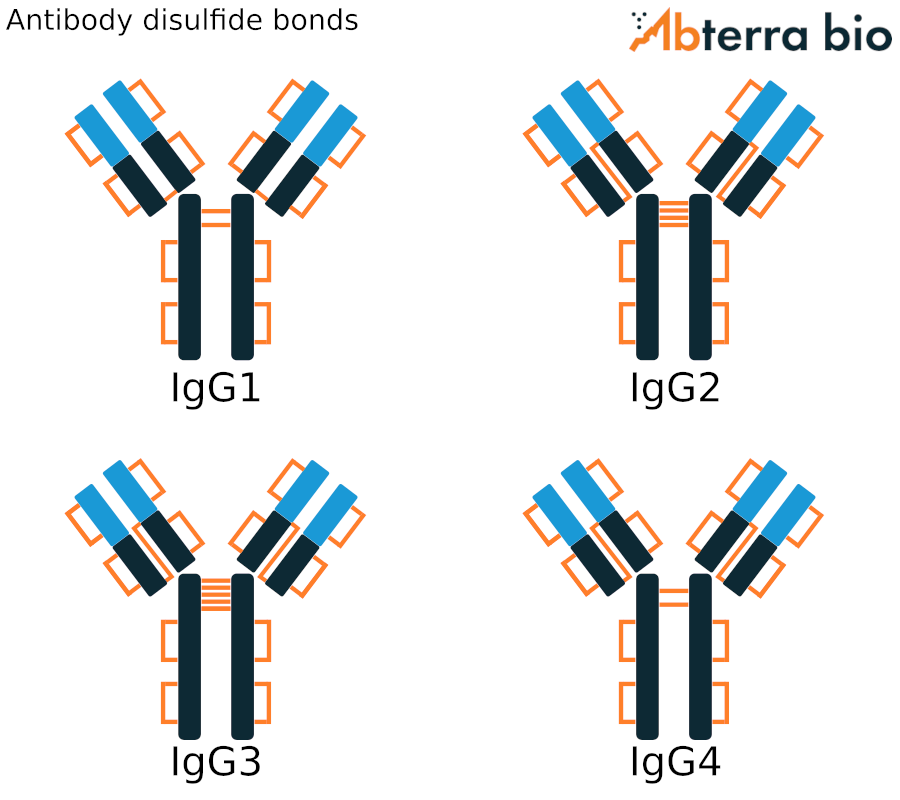
Antibody disulfide bonds
The antibody heavy and light chains are connected via interchain disulfide bonds, while intrachain bonds further constrain the antibody structure. All four IgG isotypes have twelve intrachain disulfide bondes and one bond connecting each heavy chain to a light chain. The position of this heavy-light chain bond differs between IgG1 and the other isotypes, as shown in the figure to the right.
The disulfide bonds between the pair of heavy chains is located in the ‘hinge’ region of the antibody. The number of hinge disulfide bonds range from just two in IgG1 and IgG4 to eleven in IgG3.
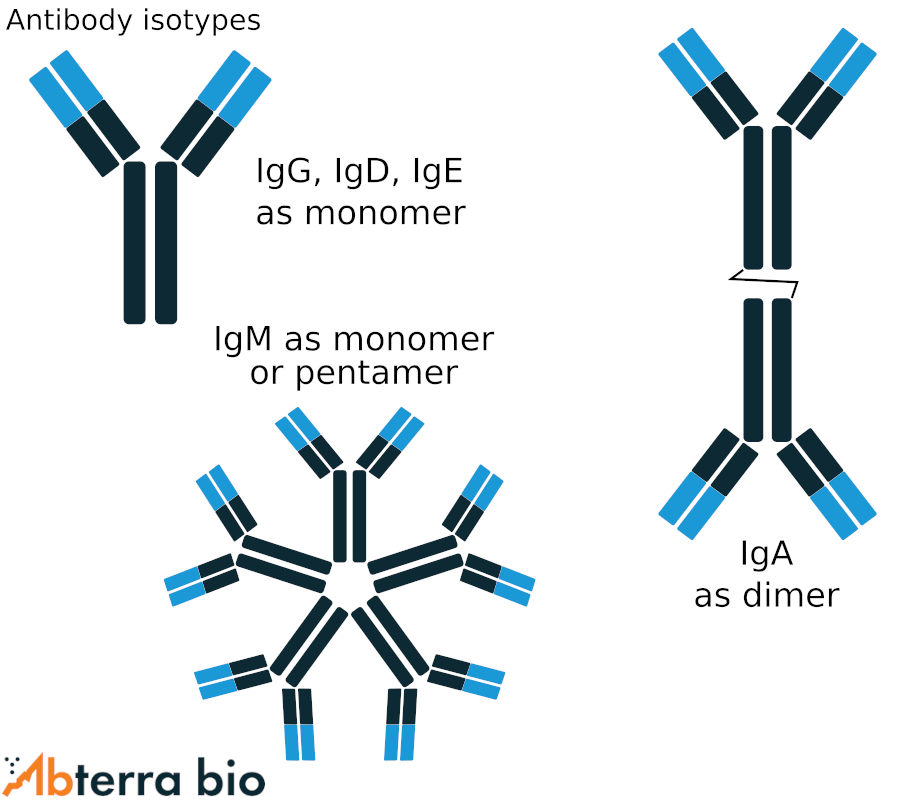
Major antibody isotypes
Antibodies are divided into five isotypes, as determined by their constant region. Many of the isotypes can be expressed as both a membrane bound form and secreted form.
Tell Us About Your Project.
Need more information? We're here to answer any questions you have.
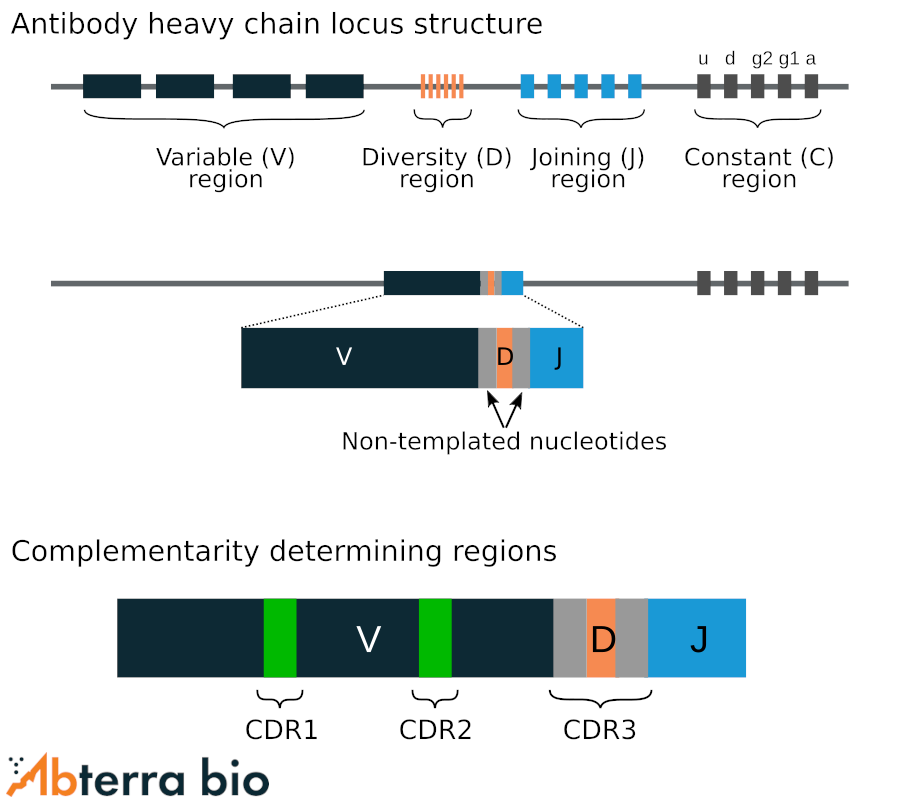
Antibody locus structure
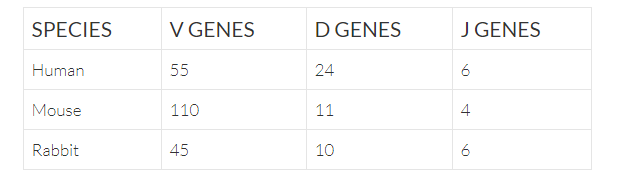
Antigen binding of antibodies is primarily defined by the complementarity determining regions (CDRs). The CDR3s of both heavy and light chains are formed by somatic recombination of the antibody heavy and light chain locii. The heavy chain (IgH) locus is formed by the recombination of one variable (V), one diversity (D), and one joining (J) gene though many of these gene segments exist at the locus. For light chain (IgK/IgL), the gene is formed by the recombination of a V and J gene.
CDRs contain hotspots for further diversification driven by somatic hypermutation.
Further reading:
Recombination centres and the orchestration of V(D)J recombination
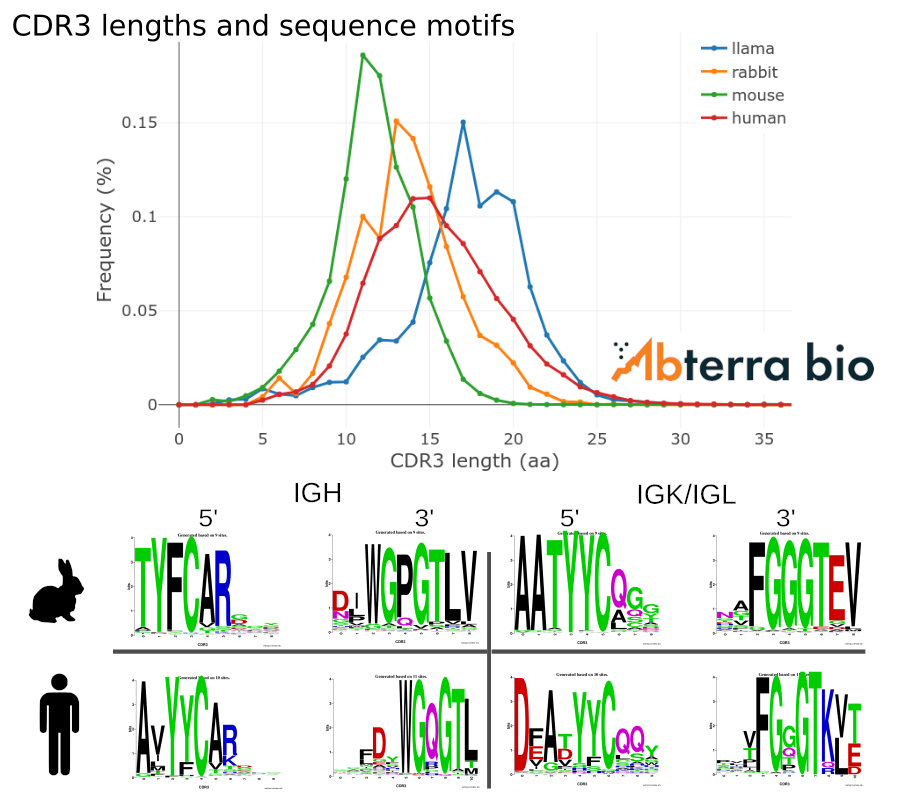
CDR3 features
CDR3 length and sequence composition varies across different organisms. While rabbit, mouse, and human antibodies are comprised of two heavy and two light chains, camelids also produce heavy-chain only antibodies (HCAbs). The length of the HCAb CDR3s are slightly longer than CDR3s from rabbit, mouse, and human.
CDR3 sequences can often be identified by their common sequence motifs at the 5′ and 3′ ends. These position weight matrices show motifs for rabbit and human.
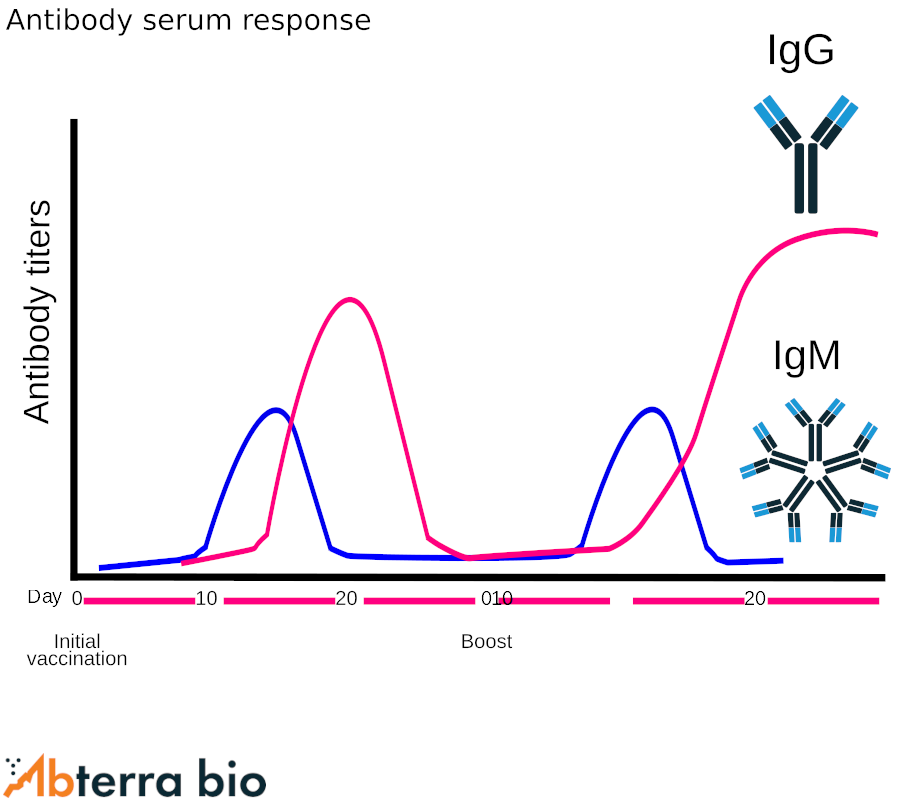
Antibody serum titers
Antibody titer is a measure of the amount of antibody present in an individual that binds a specific target. It’s typically expressed as the inverse of the highest dilution of the antibody that still delivers a positive result, often by ELISA. For example, a titer of 1000 would indicate that at a 1:1000 dilution the absorbance was significantly greater than background.
Antibody titer dynamics after immunization and boost are shown on the right. The expected serum titers for IgM and IgG are depicted.
The antibody response can vary dramatically based on age, genetic background, immunogen, and prior exposures.
Further reading:
VACCINES: PAST SUCCESSES AND FUTURE PROSPECTS. Virology Chapter 8.
Duration of Humoral Immunity to Common Viral and Vaccine Antigens
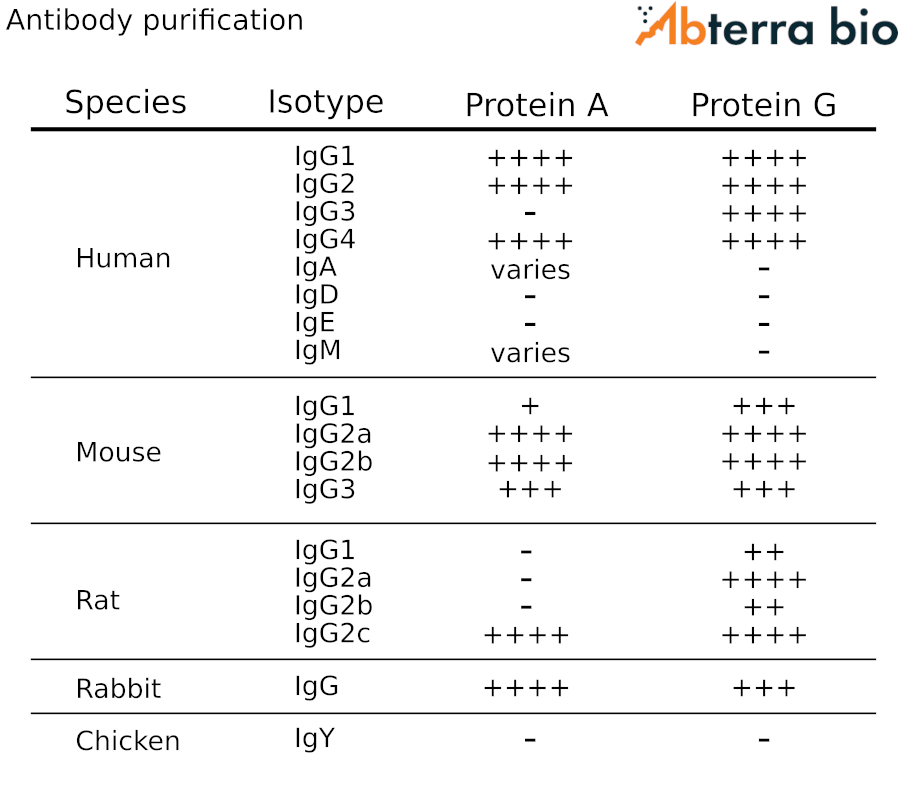
Antibody purification using Protein A/G
For purifying antibodies from diverse species and isotypes from serum, we’ve created the table at the right. The table represents the aggregation of multiple vendor guidelines and publications, together with our own experience.
For some isotypes, such as IgM, specialized reagents have been developed that are better suited for targeted enrichment.
Only a small fraction (2-5%) of circulating antibody in serum bind to a specific target, even during hyperimmunization. Antigen enrichment is the best way to purify polyclonal antibodies against a specific target.