VHH Discovery By Serum Proteomic Analysis
Llama VHH discovery from serum
Camelids, such as llamas and alpacas, naturally produce both conventional antibodies and heavy-chain only antibodies (HCAbs)(1). The antigen-binding domain of the HCAb, called the VHH or nanobody, is comprised of a single polypeptide fragment and is easily expressed in a variety of expression systems, including E. coli, in large quantities. Their single-chain nature, with extra intra-chain cysteine bridges, make them structurally robust, with high thermostability. The unique properties of VHHs make them attractive immunoreagents as well as therapeutics. VHHs have already demonstrated potential as therapeutics against difficult targets including HIV glycoproteins and enzyme binding pockets, and the first VHH-based therapeutic, caplacizumab, was approved by the FDA in 2019.
VHH discovery is traditionally performed through phage display from peripheral blood mononuclear cells of either naive or immunized camelids. In this approach, antibody transcripts are cloned from peripheral blood mononuclear cells into phages. The phage library is panned over several rounds to arrive at a collection of VHHs that have high affinity to the target. While phage display enables the screening of billions of VHHs, there are unintended biases imposed by the phages that may restrict the number of distinct VHHs recovered.
Alicanto® is a llama VHH discovery platform that identifies target-specific VHHs directly from the serum of immunized camelids. By not cloning into phage and panning, Alicanto® delivers the natural diversity of the llama immune response. Here we describe a case study of Alicanto® to simultaneously discover an anti-CD20 VHH and VHHs to the carrier protein, KLH. Below is an overview of the Alicanto® process, described more fully in this blog post.
Tell Us About Your Project
VHH discovery against a peptide
Immunization
We immunized a single adult male llama with a peptide for rabbit cell surface marker CD20 conjugated to KLH. The llama was immunized 4 times over 14 weeks, and bleeds were taken 1 week and 2 weeks after each boost and processed into peripheral blood mononuclear cells (PBMCs). At week 14, serum was isolated from the whole blood.
Antibody repertoire sequencing and analysis
Repertoire construction and analysis were performed by our antibody repertoire analysis platform, Reptor™. Reptor automatically quality filters reads, stitches read pairs to determine a full VHH sequence, and corrects errors(2). Each bleed was processed individually and the immunization time point and isotype were retained for each antibody. Roughly 128,233 unique IgG2 sequences were recovered. Limited expression of IgG3 resulted in a small repertoire for this isotype, with fewer than 2,500 distinct sequences recovered from each time point. Since few IgG3 target-specific antibodies were purified for mass spectrometry analysis, the IgG3 antibodies were omitted from further analysis. Each nucleotide sequence was translated to create an amino acid sequence database for analysis with mass spectrometry.
Each antibody was further analyzed to determine the germline V, D, and J genes that produced the clone, a process called V(D)J-labeling (3). The three complementarity-determining regions (CDRs) for each antibody were annotated. Antibodies with nearly identical CDR3s were clustered together into clone clusters.
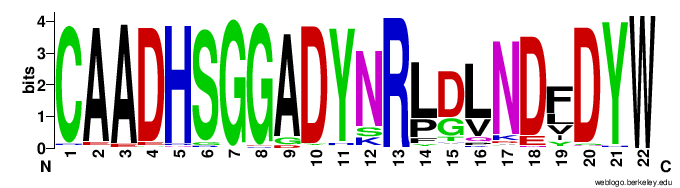
As an example, one clone cluster containing 143 distinct amino acid CDR3s across 1,470 VHH sequences can be represented as the web logo below (VDJ junction shown)
Mass spectrometry analysis
The serum antibodies were fractionated using affinity chromatography and isotype separation (1). Two samples were analyzed by Alicanto®; the anti-CD20 (aCD20) IgG2 fraction and the anti-KLH (aKLH) IgG2 fraction. For neither immunogen could sufficient quantities of IgG3 be purified for mass spectrometry analysis. Each fraction was analyzed using our standard Alicanto® mass spectrometry workflow. Briefly, the fractions are run on SDS-PAGE gel, the HCAb band was digested in gel with multiple proteases, the digested bands were then run on a nano-HPLC coupled to a ThermoFisher Fusion Lumos. In total 110,911 tandem mass spectra were generated for the aCD20 fraction and 139,795 mass spectra were generated for the aKLH fraction.
MS/MS spectra from each fraction were independently mapped to the IgG2 repertoire. Alicanto® determined the set of CDR3 sequences present in the repertoire. Four CDR3s that were clustered into two clone clusters were found targeting the CD20 peptide. The limited diversity is not unexpected due to the limited number of epitopes present on the peptide and the general observation that llama prefers to raise IgG1 antibodies over HCAbs to linear epitopes (4). From the aKLH fraction, 65 distinct CDR3s in 20 clone clusters were found. None of the aKLH CDR3s had similarity to the aCD20 CDR3s.
From the clone cluster described above, we found proteomic evidence in the aKLH fraction for 1 of the 143 unique CDR3s.
VHH candidate selection and validation
We selected candidate VHHs for validation by ELISA against the desired target as well as against the other immunogens present in the immunization. Among the 4 aPep CDR3s, we selected 2 for production and added a third VHH CDR3 for validation. Among the 65 aKLH CDR3s, we selected 10 candidates for expression, with the goal of broadly sampling the 20 clone clusters.
The three VHHs from the aPep fraction and ten VHHs from the aKLH fraction were synthesized into a pET28(a)+ vector and expressed as VHHs in SHuffle E.coli cells. The VHHs were purified and tested via ELISA for binding to the CD20 peptide and KLH. In total 2 of 3 aPep VHHs and 4 of 10 aKLH VHHs showed affinity and specificity to their intended targets. The 4 aKLH VHHs included the single member of the 143-member clone cluster described above.
Conclusion
-
Alicanto® can succesfully identify llama VHHs to multiple targets in the same immunization, including challenging targets such as peptides.
-
Proteomic information can be used to identify a lead candidate from a large family of similar sequences.
-
High-throughput sequencing of the antibody repertoire enables the identification of llama VHH clone clusters that can be mined for desireable VHH properties.
References
(1) Hamers-Casterman, C. T. S. G., et al. “Naturally occurring antibodies devoid of light chains.” Nature 363.6428 (1993): 446. (PubMed: 8502296)
(2) Safonova, Yana, et al. “IgRepertoireConstructor: a novel algorithm for antibody repertoire construction and immunoproteogenomics analysis.” Bioinformatics 31.12 (2015): i53-i61. (PubMed: 26072509)
(3) Bonissone, Stefano R., and Pavel A. Pevzner. “Immunoglobulin classification using the colored antibody graph.” Journal of Computational Biology 23.6 (2016): 483-494. (PubMed: 27149636)
(4) Pardon, Els, et al. “A general protocol for the generation of Nanobodies for structural biology.” Nature protocols 9.3 (2014): 674. (PubMed: 24577359)